Abstract
Background: The majority of the patients with low risk myelodysplastic syndrome (MDS) become RBC-transfusion-dependent and thus symptoms resulting from iron overload and oxidative stress may develop. Yet, iron-chelation has not been part of the standard treatment for these patients. The purpose of this study was to assess the effect of deferiprone (L1), an iron chelator, on oxidative stress parameters in iron overloaded and blood dependent patients with low risk MDS.
Methods: This work was supported by a grant from Apopharma.
Nineteen low risk MDS patients were enrolled. Patients were classified as very low-, low- and intermediate-risk disease, according to the Revised International Prognostic Scoring System (IPSS-R). Inclusion criteria were a cumulative transfusion burden of >20 red blood cells (RBC) units and evidence of iron overload (serum ferritin >1,000 ng/mL). Deferiprone at a dose of up to a maximum dose of 100 mg/kg was administrated for 4 months. Blood samples were taken at baseline, at each monthly visit, and at the end of the study. The samples were tested for oxidative stress parameters: reactive oxygen species (ROS), phosphatidylserine (PS), glutathione (GSH) and membrane lipid peroxidation (LP); iron parameters: serum ferritin and cellular labile iron pool (LIP), and CBC. The primary efficacy variable as a measurement of oxidative stress was ROS. Student's t-test was applied for statistical analyses.
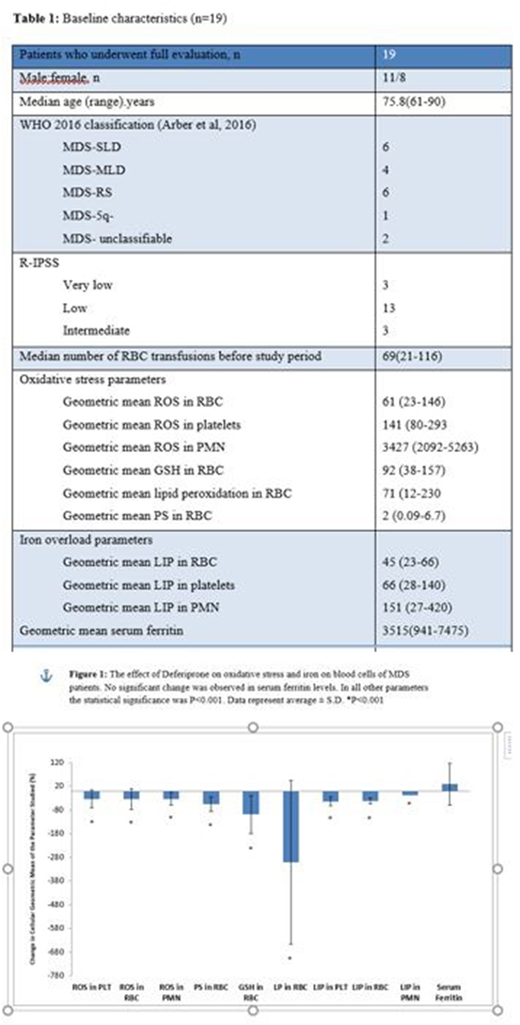
Results: Patients (n=19) were treated with deferiprone in a daily dose of 25-100 mg/kg, median of 50 mg/kg/day. Eight patients received treatment for 120 days and completed the study. One of them had mild neutropenia that resolved after short discontinuation of the study drug and finished the study with no more complications. Eleven patients did not finish the study: eight patients due to gastrointestinal (GI) adverse effects, two patients withdrew their consent (one of them after mild neutropenia that resolved) and one patient was killed in a car accident. We thus had measurements of at least two time points for 16 of the patients. The median number of RBC transfusions during study period was 11 (2-20). The primary efficacy variable ROS, as a measurement of oxidative stress, was significantly decreased, in all three lineages: platelet, RBC and neutrophils (decrease of 32%, 33% and 32% respectively, each at P=0.001). Deferiprone administration was also associated with a reduction in other cellular markers of oxidative stress in RBC: PS, GSH, LP and LIP (decrease of 54%, 98%, 300%, and 42% respectively, each at P=0.001). No significant changes were observed in serum ferritin levels, likely due to the short treatment period in patients on repeated transfusion requirements.
Conclusions: This is the first prospective study on chelation with Deferiprone in MDS patients. Our data provide preliminary evidence that administration of Deferiprone in a median daily dose of at 50 mg/kg can decrease iron-induced oxidative stress in iron-overloaded MDS patients. Of note, no events of agranulocytosis were observed. The future remains challenge is to prove that reduction in iron toxicity will eventually translate into a clinically meaningful improvement.
Prof. Rachmilewitz who unfortunately passed away last year, had a significant contribution to the conception and design of this study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.